|
|
||||||||
 |
|
The Atom Atoms are the smallest particles of an element that can enter into a chemical, non-nuclear reaction. They are made up of smaller constituents called electrons, protons, and neutrons. Protons and neutrons are found in the nucleus and are therefore collectively referred to as nucleons. Electrons orbit outside the nucleus. Each of these particles has a different charge and mass.
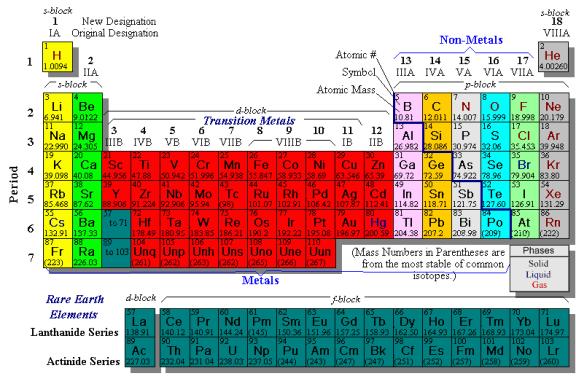
Table 1: Subatomic particle properties Each element has an atomic number, which is the number of protons in the atom; that is, the number of protons in an atom defines what element that atom is. Atoms with the same number of protons and different numbers of neutrons are called isotopes. For example, ordinary hydrogen has one proton, one electron, and no neutrons. Deuterium, or heavy hydrogen, is another isotope of hydrogen that has one proton, one electron, and one neutron. Shown below is a periodic table of the elements for your reference.
Periodic table used courtesy of ThinkQuest Binding Energy, or
Something interesting happens when a helium atom is formed. The helium atom is made up of two protons, two neutrons, and two electrons. Adding up the masses of these constituents gives us 4.0331 amu. However, we know from experimental measurement that the actual mass of a helium atom is 4.0026 amu. The difference between the calculated mass and the experimental mass is called the mass defect. So where did the 0.0305 amu go? It was converted to energy. Enter Einstein. The equation that represents the conversion of mass into energy is represented by Einstein's famous
Radioactive Decay The nucleus of an atom is stable if it cannot be changed to another configuration without adding energy from the outside. Nuclei that are not stable are radioactive. They change spontaneously (decay) to more stable nuclei. There are several types of radioactive decay that are classified by the type of radiation that is produced. 1. Alpha decay, or
2. Beta decay, or
3. Another type of beta decay,
4. Electron capture occurs when one of the electrons in an atom is captured by the nucleus and a proton is converted to a neutron. 5. Gamma emission, or
Certain types of radiation are more likely to cause negative biological effects because of the particles that they emit. Certain particles possess much higher energies than ordinary chemical bond energies, which hold molecules together within our bodies. When these particles strike and penetrate matter, they produce ions, a particle charged through the loss or gain of one or more electrons, and molecular fragments that are extremely reactive. In our bodies this can seriously disrupt the normal operations of the cells. Alpha particles are the most dangerous in this respect, about 10 times more so than beta particles, and 20 times more so than gamma rays. Nuclear Fusion Fusion is the process of combining small nuclei into large nuclei. This is the process that takes place in stars, as hydrogen atoms are fused together to form helium. As we mentioned before, there is a small amount of mass lost that is converted to a very large amount of energy during this process. However, very high temperatures are needed to give the nuclei enough energy to overcome the repulsive forces that normally keep the atoms apart. At this temperature, all of the molecules dissociate into atoms, which ionize, forming a state of matter called plasma. However, the high temperatures are a problem if you want to build a fusion reactor, because the temperatures needed to initiate fusion are so high, that mechanical devices cannot contain the plasma, because they would melt. Other methods of containment that have been studied are containment by a magnetic field and containment through the use of focused laser beams. Nuclear Fission Many heavy elements with small binding energies per nucleon decompose into more stable elements with larger binding energies per nucleon, sometimes producing neutrons. This decomposition is fission. A common example of a fission reaction: Uranium-235 is a fissile isotope of uranium; that is, the isotope of uranium that has 92 protons and 143 neutrons is fissionable. If a neutron is shot at a uranium-235 atom, the uranium-235 atom absorbs the neutron, making it uranium-236, which is unstable. The unstable atom splits into krypton-92 and barium-142, for example, and releases two more neutrons in the process. This is reaction is represented by the following equation: 1 neutron +
The two emitted neutrons can then go on to repeat the process unless absorbed by something else. Of course, in a fission reactor it is important that the chain reaction continues, but does not grow out of control. For more info visit ThinkQuest at http://library.thinkquest.org/17940/texts/atom/atom.html
|
Written and created by Cami Idzerda. Last updated 11/29/2001. Email: CIdzerda@aol.com |